
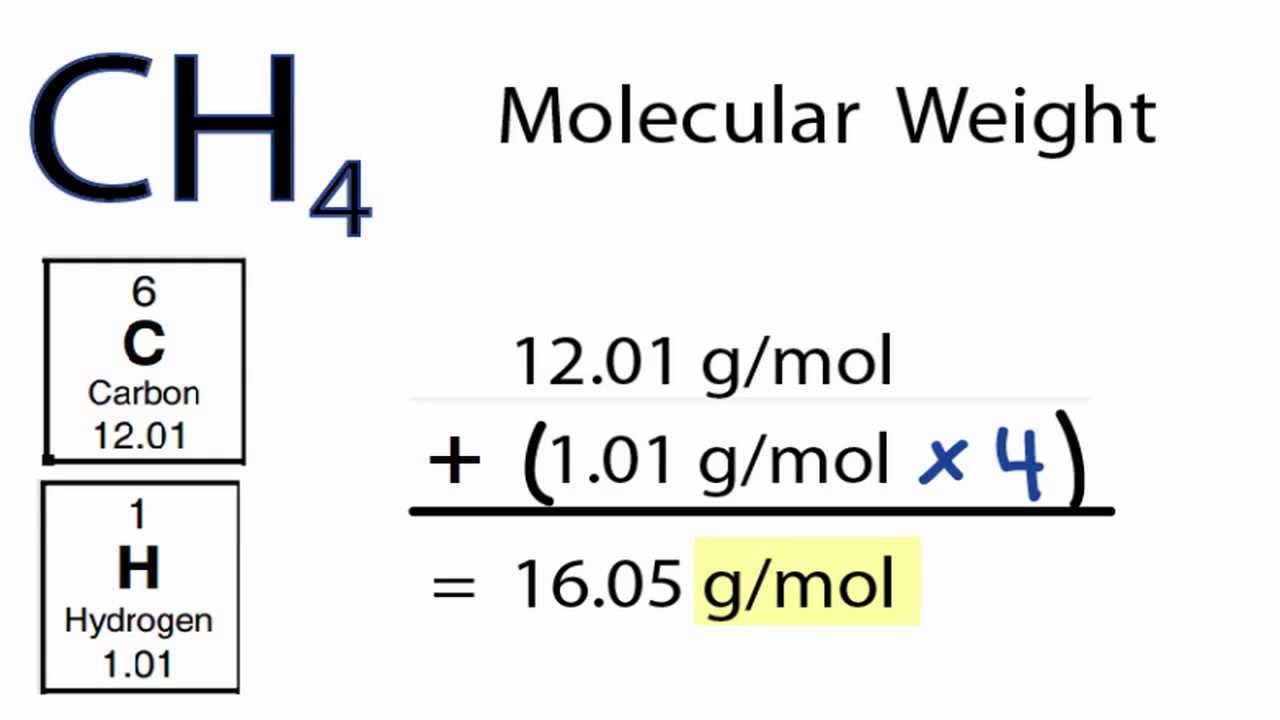
What is the molar concentration of CoCl 2♲H 2O? The solution in Figure 4.6 "Preparation of a Solution of Known Concentration Using a Solid Solute" contains 10.0 g of cobalt(II) chloride dihydrate, CoCl 2♲H 2O, in enough ethanol to make exactly 500 mL of solution. These concentrations and their units are summarized in Table 4.1 "Common Units of Concentration". For aqueous solutions at 20☌, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter. The concentrations of very dilute solutions are often expressed in parts per million ( ppm), which is grams of solute per 10 6 g of solution, or in parts per billion ( ppb), which is grams of solute per 10 9 g of solution. Each measurement can be expressed as a percentage by multiplying the ratio by 100 the result is reported as percent m/m or percent m/v.

A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution a concentration on an m/v basis is the number of grams of solute per milliliter of solution. 250 mol NaOHĬalculate the number of millimoles of alanine, a biologically important molecule, in 27.2 mL of 1.53 M alanine.Ĭoncentrations are often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications. Use either Equation 4.5 or Equation 4.6, depending on the units given in the problem.īecause we are given the volume of the solution in liters and are asked for the number of moles of substance, Equation 4.5 is more useful: moles NaOH = V L M mol/L = (2.
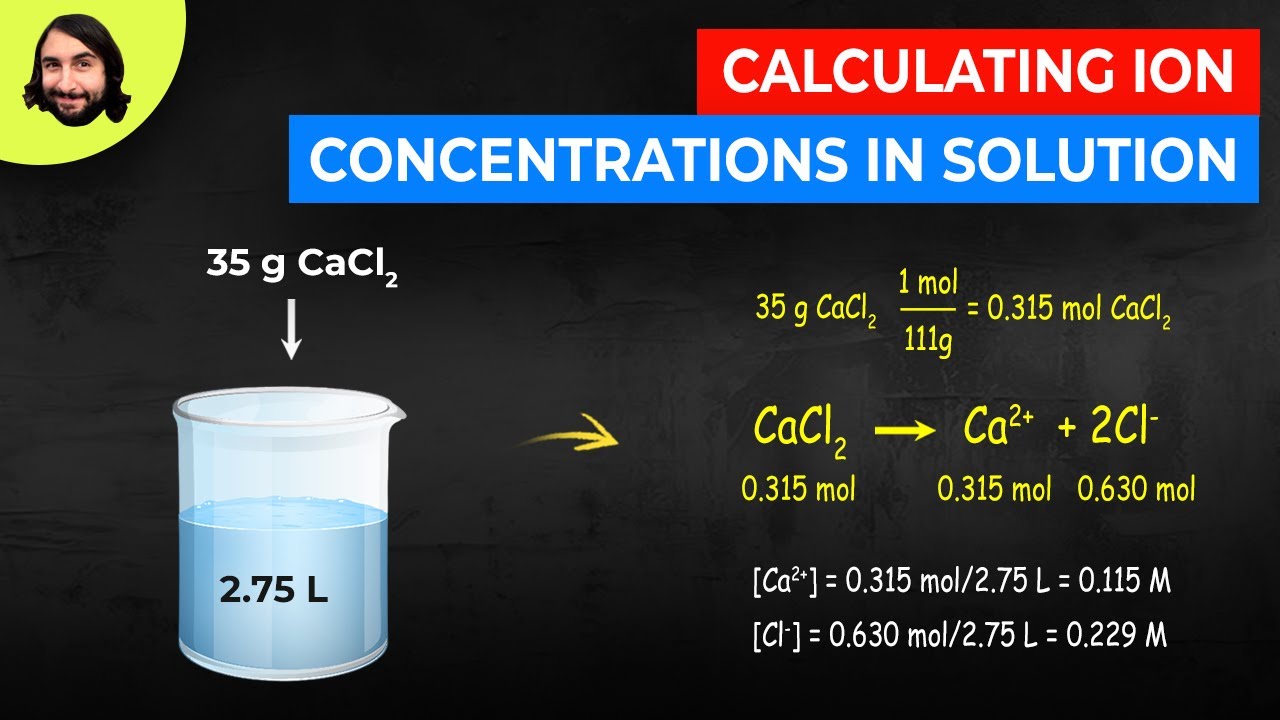
Given: identity of solute and volume and molarity of solution Calculate the number of moles of sodium hydroxide (NaOH) in 2.50 L of 0.100 M NaOH.


 0 kommentar(er)
0 kommentar(er)
